

A. S. Lubaina1*, P. R. Renjith1, Praveen Kumar2
1Department of Botany, Christian College, Kattakada, Thiruvananthapuram, Kerala, India
2Department of Zoology, Government College for Women, Thiruvananthapuram, Kerala, India
*Address for Corresponding Author:
A. S. Lubaina,
Department of Botany, Christian College, Kattakada, Thiruvananthapuram, Kerala, India.
Abstract
Objective: Pineapple peel the major by-product of pineapple processing industry represent between 50 to 65 % of total weight of the fruit. The increasing production of pineapple processed items results in massive waste generations causing severe environmental pollution. The present study aims to recycling pineapple wastes by evaluating bactericidal potentiality of petroleum ether, ethyl acetate, ethanol and water extracts of pineapple peel against infectious bacterial strains. Materials and methods: The four extracts of pineapple peel were evaluated for antibacterial activities against gram-positive bacteria such as Staphylococcus aureus (ATCC 29213)and gram-negative species such as Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Vibrio cholera (MCV09) and Klebsiella pneumoniae (ATCC 700603) using agar well diffusion method. Results: Among the tested extracts of pineapple peel, ethyl acetate showed significant antibacterial activity in all bacterial strains and the gram negative bacteria such as Pseudomonas aeruginosa, Vibrio cholera and Escherichia coli revealed the highest zone of inhibition (20mm) and the results were comparable with the synthetic antibiotic tetracycline (24mm). Conclusion: The results of the present investigation suggest that the pineapple waste can be used as potential leads to discover new drugs to control some food poisoning and other infectious bacteria.
Keywords: pineapple peel, Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholera Escherichia coli, Klebsiella pneumoniae
Introduction
Fruit wastes may cause serious environmental problems, since it accumulates in agro-industrial yards without having any significant and commercial value. Since disposal of these wastes is expensive due to high costs of transportation and a limited availability of landfills they are unscrupulously disposed causing concern as environmental problems. Furthermore, the problem of disposing by-products is further aggravated by legal restrictions. The pineapple (Ananas comosus) is one of the most important fruits in the world and is the leading edible member of the family Bromeliaceae. Large production of this fruit in world is mostly used for juice extraction in the industry which leads to produce huge amounts of residues, including peel and other segment membranes. Pineapple peel is the main by-product of pineapple processing industry and results in massive waste generations and if it is not processed properly produces odour, soil pollution and harborage for insects (Mandalari et al., 2006). In this regard, several efforts have been made in order to utilize pineapple wastes obtained from different sources. So an eco-friendly utilization of this waste is becoming more and more necessary for the production of value added products. The wastes from pineapple canneries have been used as the substrate for bromelain, organic acids and ethanol because of the presence of sugars, vitamins and growth factors (Dacera et al., 2009).
Prevention of diseases is usually achieved by the use of chemicals which have negative impacts like human health hazards and acquisition of microbial resistance to the used chemicals. Because of such concerns, the necessity to find potentially effective safer and natural alternatives is essential. Usage of plant-derived antimicrobial agents might provide opportunities to access new antibiotics and minimizing the chances resistance to pathogenic microorganisms (Voon et al., 2012). So the discovery of new-generation drugs against infections from natural products is highly desired for the development of effective and safe antibacterial agents that would be used as a complimentary with convectional medicines. So this work was an attempt made to look for substances from pineapple peel having antibacterial potentiality to kill or inhibit the growth of microorganisms. Mechanism of antibacterial agents confide in the structure and composition of the bacterial cell. Destruction of bacterial structure led to a change in bacterial metabolism which causes cell death. Antibacterial mechanism includes cell walls destruction, disruption or injury to cell membrane and inhibition of proteins and nucleic acids. In this juncture the present study aims to evaluate the antibacterial potential of pineapple peel extracted in solvents such as petroleum ether, ethyl acetate, ethanol and water against pathogenic bacteria and search for more effective antibacterial agents with the aim of discovering potentially useful active ingredients from pineapple peel that can serve as source for the synthesis of new drugs.
Materials and Methods
Plant material
The material used for the study was the peel of mauritius variety (the most popular cultivar grown in Kerala) of pineapple fruit collected from fruit processing industry at Vazhakulam, Muvattupuzha, which is the main cultivation region of pineapple in our state.
Soxhlet hot continuous extraction
Exactly 250 g pineapple peels were finely chopped, air dried in shade at room temperature, powdered and successively extracted with 100 ml of petroleum ether, ethyl acetate, ethanol and
water for eight hours using soxhlet hot continuous extraction method. The extracts were filtered and concentrated using rotary evaporator at 50°C. The yields of extracts were calculated.
Determination of antibacterial activity
Antibacterial activity of the petroleum ether, ethyl acetate, ethanol and aqueous extracts of pineapple peel (1 mg/ml) was analyzed against bacterial strains such as Staphylococcus aureus (ATCC 29213), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Vibrio cholera (MCV09) and Klebsiella pneumoniae (ATCC 700603) using agar well diffusion method (Javed et al., 2011). The microbial strains were collected from National Collection of Industrial Microorganism (NCIM), Pune, India. The Mueller–Hinton agar (Himedia, Mumbai, India) plates were prepared by pouring into sterile petri plates. The dried plates were inoculated with 0.1% inoculum suspension of each test bacteria by swabbed uniformly over the sterile agar surface and subsequently allowed to dry. In each plate, four wells of 10 mm diameter were prepared, using a sterile well borer. These four wells were loaded with 100 μl of petroleum ether, ethyl acetate, ethanol and aqueous pineapple peel extract stock respectively with 0.1 mg/ml tetracycline as positive control and DMSO (100%) as negative control. The whole sets were incubated for 24 h at 37ºC and the zone of inhibition was measured after 24 h of incubation. Each experiment was carried out in triplicates and an average diameter of the inhibition zone was recorded in terms of millimeters (Halawi et al., 2015).
Statistical analysis
A simple statistical analysis was carried out to calculate the standard deviation. Each experiment was carried out in triplicate and average mean diameter of the inhibition zone was recorded.
Results and discussion
There has been an increasing interest in the exploration of antibacterial plant products having mechanisms of action different from those of the conventional chemical drugs. This aspect is effectively evaluated by petroleum ether, ethyl acetate, ethanol and aqueous extract of a pineapple peel on selected bacterial strains. Bactericidal activity of the petroleum ether, ethyl acetate, ethanol and aqueous extract of a pineapple peel exhibited varied susceptibility against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Vibrio cholera and Klebsiella pneumonia at the concentrations tested (Table 1). The microbicidal potential of the extract was visualized as inhibition zone by treating the pathogens with the extracts and then spreading the cells on agar plates by well diffusion assay (Figure 1 a-f). Among the pathogens tested Pseudomonas aeruginosa, Escherichia coli and Vibrio cholera were the most resistant species with ethyl acetate extract of pineapple peel. The highest antimicrobial potentials were observed in the ethyl acetate extract followed by ethanol and petroleum ether extracts respectively with all tested bacterial strains. The mechanism of antibiosis indicated by synthetic antibiotic tetracycline was comparable against the entire tested bacterial isolates.
Table 1. Antibacterial activity of petroleum ether, ethyl acetate, ethanol and water extracts of pineapple peel against selected bacterial strains.
|
Microorganisms |
Pineapple peel extract (conc: 1mg/ml) |
Zone of inhibition (mm) |
|
Pseudomonas aeruginosa |
Petroleum ether |
12 ± 0.04 |
|
Ethyl acetate |
20 ± 0.12 |
|
|
Ethanol |
15 ± 0.07 |
|
|
Water |
0 |
|
|
Tetracycline |
24 ± 0.15 |
|
|
DMSO |
0 |
|
|
Staphylococcus aureus |
Petroleum ether |
0 |
|
Ethyl acetate |
16 ± 0.08 |
|
|
Ethanol |
13 ± 0.06 |
|
|
Water |
0 |
|
|
Escherichia coli |
Petroleum ether |
12± 0.04 |
|
Ethyl acetate |
20± 0.12 |
|
|
Ethanol |
13± 0.06 |
|
|
Water |
0 |
|
|
Vibrio cholerae |
Petroleum ether |
0 |
|
Ethyl acetate |
20 ± 0.12 |
|
|
Ethanol |
17± 0.10 |
|
|
Water |
0 |
|
|
Klebsiella pneumoniae |
Petroleum ether |
11± 0.02 |
|
Ethyl acetate |
16± 0.08 |
|
|
Ethanol |
11± 0.02 |
|
|
Water |
0 |
Values of zone of inhibition in mm are mean ± SD of three independent replications; 0 = No zone of inhibition.
The effectiveness of an antibacterial agent is measured by its ability to inhibit and kill bacteria. The antibacterial activity revealed by the extracts might be due to presence of polyphenols, flavonoids, saponins and other secondary metabolites present in the extract. Flavonoids and polyphenols are more potent in inhibiting gram-positive bacteria. Both are phenolic compounds which have polar properties mostly work in the peptidoglycan layer in gram-positive bacteria rather than the non-polar lipid layer. Most plant phenolic compounds are non-toxic for human consumption therefore they could be used to prevent growth of many food-borne and food spoilage microorganisms. These phenols have the ability to denatures the protein and its lipophilic nature helps them to attract the lipid molecules contained in the cell membranes and damage the bacterial cell membrane (Maurer, 2001).
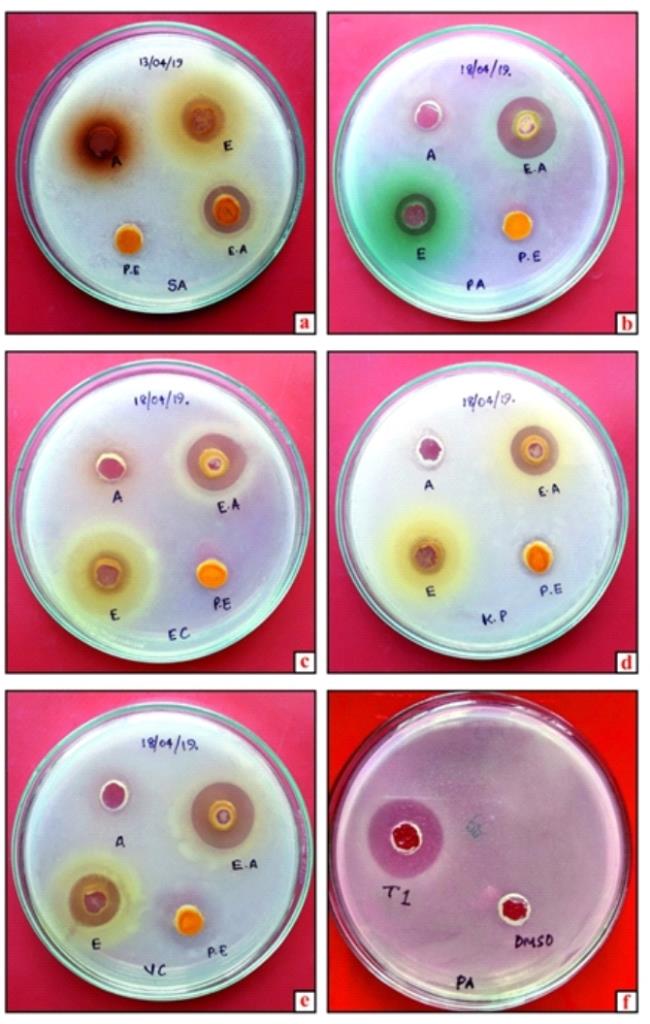
Figure 1 (a-f). Antibacterial activity of petroleum ether, ethyl acetate, ethanol and water extracts of pineapple peel against a. Staphylococcus aureus (SA), b. Pseudomonas aeruginosa (PA), c. Escherichia coli (EC), d. Klebsiella pneumonia (KP), e. Vibrio cholera (VC), f. positive control (Tetracycline) and negative control (DMSO) against PA.
Both bromelain and saponins act on bacterial cell wall and membranes. The active compound working against gram-negative bacteria is mainly bromelain. Bromelain is a proteolytic enzyme play a role in the breakdown of proteins, one of the essential component in bacterial membrane causing injury and cell death. It also disintegrate protein in surface membrane which eventually weakens the cell wall, leads to cell leakage and damages the cell. The numbers of amino acids in the bacterial cell wall are thought to determine the antibacterial activity of proteolytic enzymes. Saponins increases the permeability of the bacterial cell membrane, causing alteration in the structure and function of the membrane thereby disrupting the surface tension of the cell wall and allowing antibacterial substances to easily enter the cells and interfere the cell metabolism which in turn leads to the lysis of membrane. Saponins selectively interact with cholesterols on cell membrane leaving a hole in the membrane (Eshamah et al., 2013).
Due to the variation in composition of active compounds in various extract of pineapple peel resulted in significant difference on the level of bactericidal activity (inhibitory zone) against the tested bacterial strains. The mechanism of antimicrobial activity of phenolic compounds is primarily by its ability to act as non-ionic surface-active agent, therefore disrupting the lipid - protein interface or by the denaturation of proteins and inactivation of enzymes in the pathogens. Secondly, phenols alter the permeability of the membrane that could result in the uncoupling of oxidative phosphorylation, inhibition of active transport, and loss of metabolites due to membrane damage. Gallic acid has proven antifungal and antibacterial properties (Boudet, 2007). Polyphenols inhibit bacterial DNA gyrase by binding to the ATP binding site (Korir et al., 2012). Similarly, tannins exert antimicrobial activities by iron deprivation, hydrogen binding or specific interactions with vital proteins, such as enzymes in microbial cells, bind to adhesions, complex with cell wall, other membranes and metal ion complexes. Flavonoids are also possessing antimicrobial potential by link to adhesions or complexes with the cell wall, inactivation of enzymes and inhibition of HIV reverse transcriptase (Akinpelu et al., 2008).
Conclusion
The antibacterial potential exhibited by ethyl acetate extract of pineapple peel was effective against gram-positive and gram-negative bacterial strains. The results of the present study encourage for the search of newer active compounds from pineapple peel responsible for its antibacterial potential. From the results it can be concluded that pineapple peel could be a useful source for antibiotic production and has the potential of medicinal and industrial application. Antimicrobial properties of various extracts from pineapple peel have recently been of great interest because of their possible use as natural additives emerged from a growing tendency to replace synthetic antimicrobials with natural ones.
Acknowledgements
This work was supported by the Kerala State Council for Science Technology and Environment, Thiruvananthapuram, Kerala (Order No. 355/2018/KSCSTE dt 14-08-2018).
Conflict of interest
The authors are declaring no conflict of interest.
References
Akinpelu DA, Adegboye MF, Adeliye OA, Okoh AI. 2008. Biocidal activity of partially purified fractions from mathanolic extract of Garcinia kola (Heckel) seeds on bacterial isolates. Biological Research, (41): 277-287.
Boudet AM. 2007. Evolution and Current Status of Research in Phenolic Compounds. Phytochemistry, (68): 2722-2735.
Dacera DM, Babel S, Parkpian P. 2009. Potential for land application of contaminated sewage sludge treated with fermented liquid from pineapple wastes. Journal of Hazardous Materials, (167): 866-872.
Eshamah H, Han I, Naas H, Rieck J, Dawson P. 2013. Bactericidal Effects of Natural Tenderizing Enzymes on Escherichia Coli and Listeria monocytogenes. Journal of Food Research, 2 (1):8-18.
Halawi MH, Rahman SMA, Yusef H. 2015. Comparative study of the antifungal activity of Olea europaea L. against some pathogenic Candida albicans isolates in Lebanon. International Journal of Current Microbiology and Applied Sciences, 4(6): 970-984.
Javed S, Javaid A, Mahmood Z, Javaid A, Nasim F. 2011. Biocidal activity of citrus peel Essential oils against some food spoilage bacteria. Journal of Medicinal Plants Research, (5): 3697-3701.
Korir RK, Mutai C, Kiiyukia C, Bii C. 2012. Antimicrobial activity and safety of two medicinal plants traditionally used in Bomet district of Kenya. Research Journal of Medicinal Plant, (6): 370-382.
Mandalari G, Bennett RN, Bisignano G, Saija A, Dugo G, Faulds CB, Waldron KW. 2006. Characterization of flavonoids and pectin from bergamot (Citrus bergamia Risso) peel, a major by product of essential oil extraction. Journal of Agricultural and Food Chemistry, (54): 197-203.
Maurer HR. 2001. Bromelain: Biochemistry, Pharmacology and Medical use. Cellular and Molecular Life Sciences, 58(9): 1234-45.
Voon HC, Bhat R, Gulam R. 2012. Flower extracts and their essential oils as potential antimicrobial agents. Comprehensive Reviews in Food Science and Food Safety, 11(1):34-55.
gaziantep escort istanbul escort izmir escort konya escort istanbul escort taksim escort