

Mahendra A. Giri1*, Rasika D. Bhalke2, K. Vanitha Prakash3, Sanjay B. Kasture4
1Department of Pharmacology, Sanjivani College of Pharmaceutical Education and Research, Kopargaon, Maharashtra, India 423603
2Department of Pharmacognosy, Sanjivani College of Pharmaceutical Education and Research, Kopargaon, Maharashtra, India 423603
3Department of Pharmaceutics, Shri Sai Jyoti College of pharmacy, Vattinagula Pally, Gandhipeth, Hydrabad-500075 Telangana, India
4Department of Pharmacology, Pinnacle Biomedical Research Institute, Bhopal, Madhya Pradesh, India
*Address for Corresponding author
Mahendra A. Giri
Department of Pharmacology,
Sanjivani College of Pharmaceutical Education and Research, Kopargaon, Maharashtra, India 423603
Abstract
Objective: In the present study, comparative antioxidant and antiparkinsonian effect of hydroalcoholic extract of Nardostachys jatamansi (HENJ) and Mucuna pruriens (HEMP) was studied. Materials and methods: The antiparkinsonian activity was evaluated by using haloperidol induced catalepsy, reserpine induced hypolocomotion, tacrine induced vacuous chewing movements, orofacial brusts and tongue protrusion. Antioxidant activity was assessed by using DPPH radical scavenging assay and H2O2 scavenging assay. The results were analyzed by repeated measure ANOVA followed by Dunnett's test. Results: Mixture 30 and 100 mg/kg shows extremely significant improvement in haloperidol induced catalepsy, reserpine induced hypolocomotion and tacrine induced vacuous chewing movements, orofacial burst and tongue protrusion as compare with HENJ and HEMP shows significant improvements in dose dependent manner. In DPPH and H2O2 scavenging assay, both the extracts exhibited free radical scavenging activity. In DPPH assay the IC50 value of ascorbic acid, HENJ, HEMP and Mixture (1:1) was 18.15, 211.54, 114.85 and 109.12μg/ml respectively whereas in H2O2 assay it was 28.58, 146.58, 98.18 and 179.47μg/ml respectively. Conclusion: Hydroalcoholic extracts has showed almost significant protections but mixture of extracts has shown extremely significant protection against haloperidol induced catalepsy, tacrine induced vacuous chewing movements, orofacial burst, tongue protrusion and reserpine induced hypolocomotion.
Keywords: Antiparkinsons, antioxidant, Nardostachys jatamansi, Mucuna pruriens, haloperidol, tacrine, reserpine
Introduction
In neurodegenerative diseases second most common age related disorder is Parkinsons Disease, which affects more than 10 million people worldwide. Rate of diagnosis of Parkinsonism disease increases with age from that 4 percent of Parkinsons disease are diagnosed before age 50 and in peoples older than age 80 it is stable (Naqvi, 2018). Neuronal death in substantial nigra, mitochondrial respiratory failure and increased oxidative stress are the common manifestations in Parkinsons disease (Kosaraju et al., 2014). Dopamine neuron degeneration in substantia nigra pars compacta region causes abnormal activities of dopamine within basal ganglia circuit’s results in muscle rigidity and catalepsy which is the sign of Parkinsons disease (Adedeji et al., 2014). When the nigrostriatal dopamine system activity is reduced causes dystonia, one of the important features of Parkinsons Disease, dopamine receptor blockers can cause acute and tardive dystonia and drug induced parkinsonism (Shetty et al., 2019). Due to dysregulation in sensory system, pain starting from central to peripheral including polyneuropathy is a heterogeneous symptom in parkinsons disease (Tai et al., 2020). In parkinsons disease bradykinesia, tremors, stiffness of limbs and torso, and postural instability are the four main symptoms from which at least two need to present for epidemiological study of parkinsons disease. Due to dopaminergic cell degeneration excessive activation of ionotropic Glu receptors causes overexcitement of high concentration of Glu and damage neurons called as excitotoxicity, microglial activation, oxidative stress, neuroinflammation and mitochondrial dysfunction leads to apoptosis (Ray et al., 2018; Aware et al., 2017).
Mucuna pruriens L. (Family: Fabaceae) (MP) have immense medicinal properties specifically due to high L-DOPA (L-3,4-dihydroxyphenylalanine) content and its role in parkinsons disease (Rane et al., 2019). MP belonging to family Fabaceae which preferably found in tropical and subtropical regions of world. MP are used traditionally since 1500 BC to treat Parkinson disease. Varity of components are present in seeds of Mucuna pruriens other than L-DOPA susch as proteins, lipids, dietary fibers and carbohydrates, minerals such as sodium, potassium, calcium, magnesium, iron, zinc, copper, manganese and phosphorus (Cassani et al., 2016; Kasture et al., 2013).
Nardostachys jatamansi (NJ) commonly named as jatamansi belongs to family Valerianaceae shows presence of terpenes, saponins, glycosides, flavonoids, tannins and phenolic compounds which are responsible for synergistic reduction in oxidative stress via inhibition of mono-amine oxidase enzymes (Patil et al., 2012). Sequiterpines, mainly Jatamansone, and coumarins are main active constituents present in NJ including other sequiterpines such as Alpha-patcho-ulense, Beta- eudesemo, beta-sitosterol, elemol, angelicin, jatamansin, jatamansinol, calarene, beta-atchoulense, n-hexaco-sanyl, n-hexacosane, Oroselol, valeranal, valeranone, seychelane, nardostachnol, nardostachone and also volatile oil (Nakoti et al., 2017; Mishra et al., 2014). Traditionally Nardostachys jatamansi used for tonic, stimulant, and antiseptic effect purpose and have antibacterial, antifungal, antiviral, antioxidant potentials alongside used in nervy headache, menopausal symptoms, flatulence, epilepsy, hyperlipidemia and intestinal colic (Mishra et al., 2014).
Materials and Methods
Experimental Animals
Experiments were performed using male Wistar rats weighing 180-200g and Balb/c mice 20-25g. Animals were maintained at 22°C ± 2°C on a standard pellet diet and water ad libitum. Institutional Animal Ethics Committee for Animal Experiments of Sanjivani College of Pharmaceutical Education and Research, Kopargaon approved the study under the protocol SCPER/CPCSEA/IAEC/2019-20/01 and all experiments were conducted in accordance with guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Behavioral tests were performed during the light cycle between 10.00 a.m. and 4.00 p.m.
Drugs and Chemicals
Tacrine and Reserpine were purchased from Sigma, Aldrich, Mumbai. All other chemicals used were of analytical grade and purchased from standard manufacturer.
Plant material and extraction
Dry powder of Nardostachys jatamansi roots and Mucuna Pruriens beans was purchased from local market and were authenticated from department of Pharmacognosy, Sanjivani College of Pharmaceutical Education and Research, Kopargaon.
Hydroalcoholic extracts were prepared using Soxhlet’s extractor. The extracts were filtered and dried. Extracts were subjected to phytochemical screening (Hamdy, 2009). The extracts were administered in doses of 30 and 100 mg/kg (p.o.). Control group was given only vehicle in equivalent volume of plant extract.
Experimental design
Animals were randomly divided into six groups of 5 animals each. Group I-Control, Group II-Haloperidol (0.5 mg/kg) or Reserpine (1 mg/kg) or Tacrine (5 mg/kg), Group III-HENJ (30 mg/kg), Group IV-HENJ (100 mg/kg), Group V-HEMP (30 mg/kg), Group VI-HEMP (100 mg/kg), Group VII-1:1 Mixture (30 mg/kg) and Group VIII-1:1 Mixture (100 mg/kg). These eight groups were used for treatment of Parkinson’s symptoms.
Assessment of anti-parkinsonian activity
Haloperidol induced catalepsy
Male Wistar rats (weighing 180–200 g) were divided into eight groups of six each. Rats were pretreated with vehicle, HENJ (30 and 100 mg/kg, p.o.), HEMP (30 and 100 mg/kg, p.o.), 1:1 HENJ+HEMP (30 and 100 mg/kg, p.o.) and L-DOPA (30 mg/kg, p.o.) 30 min before haloperidol (0.5 mg/kg, intra-peritoneally). Catalepsy was measured at 0, 30, 60, 90, 120, 150 and 180 min time duration after haloperidol administration using bar test. Both the forepaws of the animals were placed on a wooden bar raised up 3 cm above the ground. The cutoff time (time for which animal was placed on elevated bar) was 300 seconds (Nair et al. 2007; Somani et al. 1999).
Tacrine induced jaw movements
The wire mesh floored observation chamber consisted of a clear Plexiglas box measuring 28×28×28 cm3. The box was elevated 42 cm from the surface of the table, allowing behavioral observation from all angles. Rats were divided into groups and treated with vehicle, HENJ (30 and 100 mg/kg, p.o.), HEMP (30 and 100 mg/kg, p.o.), and 1:1 HENJ+HEMP (30 and 100 mg/kg, p.o.). After 20 min, tacrine (5 mg/kg i.p.) was administered and the number of chewing movements, orofacial bursts and tongue protrusions were measured every ten min for 60 min. (Naidu and Kulkarni, 2001; Crowley et al., 2012).
Reserpine-induced hypolocomotion
Reserpine was injected intraperitoneally at a dose of 1 mg/kg in a suspension with Tween-80, 1 h after the mice were treated with vehicle, HENJ (30 and 100 mg/kg, p.o.), HEMP (30 and 100 mg/kg, p.o.), and 1:1 HENJ+HEMP (30 and 100 mg/kg, p.o.). The effect of the study compounds on reserpine-induced hypolocomotion was assessed using Actophotometer (Dolphine, India). The locomotor activity of the animals was measured for 2 min, at 2 h, 3h and 4h after reserpine had been administered (Fernandes et al., 2012).
Antioxidant activity
DPPH scavenging assay
The free radical scavenging activity of the HENJ and HEMP was measured in terms of hydrogen donating or radical scavenging ability using the stable free radical DPPH. 0.1 mM solution of DPPH in methanol was prepared and 1 ml of this solution was added to 3 ml of HENJ and HEMP solution in water at various concentrations (2-1000 μg/ml). The mixture was incubated for 45 min at room temperature and the absorbance was measured at 517 nm against the corresponding blank solution. Ascorbic acid was used as reference standard. Percentage inhibition of DPPH free radical was calculated using the following equation:
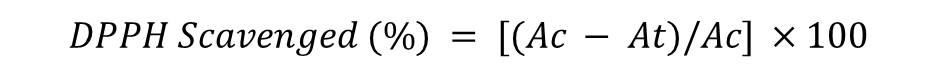
Where Ac was the absorbance of the control, and At was the absorbance of the extract or reference standard. The antioxidant activity was expressed as IC50. The IC50 value was defined as the concentration in μg/ml of the extract that inhibits the formation of DPPH radicals by 50% (Farooq and Sehgal, 2018; Pal et al., 2015; Patil et al., 2011).
H2O2 scavenging activity
A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). 3.4 ml (16 – 1000 μg/ml) extract in phosphate buffer were added to H2O2 (0.6 ml, 40 mM). Absorbance was determined at 230 nm after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide. The percentage of H2O2 scavenging of HENJ and HEMP and ascorbic acid (reference standard) was calculated as:

Where Ac was the absorbance of the control, and At was the absorbance of the sample or standard. The antioxidant activity was expressed as IC50 (Farooq and Sehgal, 2018; Pal et al., 2015; Patil et al., 2011).
Statistical analysis
Results were expressed as mean ± SEM. Significant differences between groups were determined by analysis of variance test followed by Dunnett's test.
Results
Haloperidol Induced Catalepsy
At a dose of 0.5 mg/kg i.p., haloperidol produced a significant cataleptic response. Significant reduction in haloperidol-induced catalepsy was observed in the mixture of HENJ and HEMP in the proportion of 1:1 at the doses of 30 mg/kg and 100 mg/kg when given orally. Mixture in the dose of 30 mg/kg and 100 mg/kg dose shows significant reduction in duration of catalepsy at 30 min, 60min, 90 min and 180 min as shown in figure 1.

Figure 1. Haloperidol induced catalepsy. Effect of HENJ, HEMP and its combination on Haloperidol induced catalepsy. All the values are expressed as mean ± SEM; n=5, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 significant compared to control (repeated measure ANOVA followed by Dunnett’s test)
Tacrine induced vacuous chewing movements
In tacrine induced vacuous chewing model, pretreatment with HEMP, HENJ and mixture was significantly reduces tacrine induced vacuous chewing movements but HENJ at 30mg/kg is more significant than HEMP and mixture as shown in figure 2. Similarly, HENJ and mixture was more effective than HEMP in reducing tacrine induced vacuous chewing movements. As compare to control group other all the groups are showing nearby extremely significant reduction in to vacuous chewing movement as shown in figure 2.

Figure 2. Tacrin induced vacuous chewing movements. Effect of HENJ, HEMP and its mixture in Tacrin induced vacuous chewing movements. All the values are expressed as mean ± SEM; n=5, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 significant compared to control (repeated measure ANOVA followed by Dunnett’s test)
Tacrine induced orofacial brusts
In tacrine induced orofacial burst HENJ and mixture shows extremely significant reduction in orofacial brusts. As compare with HENJ 30 mg/kg, HENJ 100 mg/kg has shown more prominent reduction in dose dependent manner. HEMP 30 mg/kg and 100 mg/kg reduces orofacial brusts but mixture shows synergistic effect when HENJ combine with HEMP as shown in figure 3.

Figure 3. Tacrine induced orofacial bursts. Effect of HENJ, HEMP and its mixture in Tacrine induced orofacial bursts. All the values are expressed as mean ± SEM; n=5, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 significant compared to control (repeated measure ANOVA followed by Dunnett’s test)
Tacrine induced tongue protrusion
In tacrine induced tongue protrusion HENJ and mixture shows extremely significant reduction in tongue protrusion. HEMP shows significant reduction at various time interval but when HEMP is given in combination with HENJ will show synergistic effect and provide extremely significant reduction in tongue protrusion. As compare with mixtures HENJ 100 mg/kg shows more effective and extremely significant reduction in tongue protrusion as shown in figure 4.

Figure 4. Tacrine induced tongue protrusion. Effect of HENJ, HEMP and its mixture in Tacrine induced tongue protrusion. All the values are expressed as mean ± SEM; n=5, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 significant compared to control (repeated measure ANOVA followed by Dunnett’s test)
Reserpine Induced Hypolocomotion
HEMP, HENJ and mixture at 100mg/kg significantly reduced reserpine induced hypolocomotion when measured for 2 min after 2h, 3h and 4h of reserpine administration, but results reveals that HEMP at (100mg/kg) is more effective than HENJ and mixture as shown in figure 5.

Figure 5. Reserpine induced hypolocomotion. Effect of HENJ, HEMP and its mixture in reserpine induced hypolocomotion. All the values are expressed as mean ± SEM; n=5, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 significant compared to control (repeated measure ANOVA followed by Dunnett’s test)
Antioxidant activity
DPPH scavenging assay
Mixture of HENJ & HEMP has shown significant DPPH scavenging activity. The scavenging effect of mixture and HEMP was comparable to ascorbic acid. The IC50 value of ascorbic acid, HENJ, HEMP and Mixture (1:1) was 18.15, 211.54, 114.85 and 109.12 μg/ml respectively as shown in figure 6.

Figure 6. DPPH scavenging assay. Percent inhibition shown by HENJ, HEMP and its mixture in DPPH scavenging assay. All the values are expressed as mean ± SD
H2O2 scavenging activity
Mixture of HENJ & HEMP shown strong H2O2 scavenging activity. The scavenging effect of mixture and HENJ was comparable to ascorbic acid. The IC50 value of ascorbic acid, HENJ, HEMP and Mixture (1:1) was 28.58, 146.58, 98.18 and 179.47 μg/ml respectively as shown in figure 7.

Figure 7. DPPH scavenging activity. Percent inhibition shown by HENJ, HEMP and its mixture in H2O2 scavenging assay. All the values are expressed as mean ± SD
Discussion
Dopaminergic D2 receptors are playing vital role in catalepsy. Haloperidol, D2 receptor blocker or dopamine deficiency produces catalepsy (Nair et al., 2007; Patil et al., 2012). We observed haloperidol significantly increases catalepsy in mice. HENJ (30, 100 mg/kg), HEMP (30, 100 mg/kg) and 1:1 combination Mixture (30, 100 mg/kg) significantly inhibited haloperidol induced catalepsy. As compare with the control group 1:1 Mixture (30, 100 mg/kg) shows extremely significant changes. The effect was observed due to the presence of levodopa, Jatamansone and Coumarinis as active chemical constituents present in the extracts along with supportive constituent and their antioxidant properties (Cassani et al., 2016; Kasture et al., 2013).
Centrally activation of muscarinic receptor shows increased vacuous jaw movements and purposeless chewing while activation in ventrolateral shows excitotoxicity and oxidative stress (Mohan et al., 2015; Gunne and Andren, 1993). Tacrine (Cholinomimetic) increases acetylcholine levels in ventrolateral regions and produces excitotoxicity and oxidative stress which results in orofacial dyskinesia along with parkinsonian symptoms (Tsai et al., 1998; Mohan et al., 2015). In the present study, tacrine significantly induced orofacial dyskinesia (increased vacuous chewing movement, orofacial brusts, tongue protrusion and yawning) and HENJ, HEMP, 1:1 Mixture in dose of 30 mg/kg, 100 mg/kg shows significantly reduction in tacrine induced changes. As compare with the control and individual doses mixture shows extremely significant reduction in tacrine induced changes.
In present study we observed that HEMP (100 mg/kg) shows extremely significant improvement in reserpine induced hypolocomotion activity followed by Mixture (100 mg/kg) and HENJ (100 mg/kg). Hedgecock et al. (2019) have reported that reserpine significantly reduces levels of dopamine, norepinephrine and 5-hydroxy tryptamine due to catabolism by monoamine oxidase enzymes, resulted in akinesia. As per study of Lieu et al. (2012); Aware et al. (2017), L-dopa (dopamine precursor) is an active chemical constituent and supportive constituents present in mucuna prurins fulfills the requirement of dopamine, resulted in improved motor action induced by reserpine significantly. Sahu et al. (2016) was reported that Sesquiterpenes, jatamansone, nardostachone are major constituents in Nardostachys jatamansi and drug induced decreased locomotor activity was significantly restored.
Sharma and Pal, (2012) has reported free radical scavenging, reducing power and metal chelating activity of Nardostachys jatamansi. The antioxidant activity might be attributed to its polyphenolic contents and phytochemical constituents (Sharma and Pal, 2012).
Mukherjee et al. (2017) have shown significant increase in OH- radical scavenging, H2O2 protection activities, and increase in enzymatic and non-enzymatic antioxidant defense systems, and in agreement with a decreased ROS levels, reduction of lipid peroxidation, after Mucuna extract treatment.
Acknowledgement
The authors are thankful to the Institute of Sanjivani College of Pharmaceutical Education and Research, Kopargaon, for providing the necessary facilities for carry out our research work in the current format.
Ethics Statement
Experiments were performed using male Wistar rats weighing 180-200g and Balb/c mice 20-25g. Institutional Animal Ethics Committee for Animal Experiments of Sanjivani College of Pharmaceutical Education and Research, Kopargaon approved the study under the protocol SCPER/CPCSEA/IAEC/2019-20/01.
Conflict of Interest statement
All the authors declare that they have no Conflict/Competing interests.
References
Adedeji HA, Ishola IO, Adeyemi OO. 2014. Novel action of metformin in the prevention of haloperidol-induced catalepsy in mice: Potential in the treatment of Parkinson’s disease? Progress in Neuro-Psychopharmacology & Biological Psychiatry 48: 245–251.
Aware C, Patil R, Gaikwad S, Yadav S, Bapat V, Jadhav J. 2017. Evaluation of L-dopa, proximate composition with in vitro anti-inflammatory and antioxidant activity of Mucuna macrocarpa beans: A future drug for Parkinson treatment. Asian Pacific Journal of Tropical Biomedicine 7(12): 1097–1106.
Cassani E, Cilia R, Laguna J, Barichella M, Contin M, Cereda E. 2016. Mucuna pruriens for Parkinson’s disease: Low-cost preparation method, laboratory measures and pharmacokinetics profile. Journal of the Neurological Sciences 365: 175–180.
Crowley JJ, Adkins DE, Pratt AL, Quackenbush CR, Van Den Oord EJ, Moy SS. 2012. Antipsychotic-induced vacuous chewing movements and extrapyramidal side effects are highly heritable in mice. Pharmacogenomics Journal 12(2): 147–155.
Farooq S, Sehgal A. 2018. Current Research in Nutrition and Food Science Antioxidant Activity of Different Forms of Green Tea : Loose Leaf, Bagged and Matcha. Current Research in Nutrition and Food Science Journal 06(1): 4–9.
Fernandes VS, Santos JR, Leao AHFF, Medeiros AM, Melo TG, Izidio GS. 2012. Repeated treatment with a low dose of reserpine as a progressive model of Parkinson’s disease. Behavioural Brain Research 231(1): 154–163.
Gunne LM, Andren PE. 1993. An animal model for coexisting tardive dyskinesia and tardive parkinsonism: A glutamate hypothesis for tardive dyskinesia. Clinical Neuropharmacology 16(1): 90–95.
Hamdy E. 2009. Trease and Evans Pharmacognosy. 16th edn, Sunders, London, pp 121-135.
Hedgecock T, Phillips A, Ludrick B, Golden T, Wu N. 2019. Molecular Mechanisms and Applications of a Reserpine-Induced Rodent Model. SSR Institute of International Journal of Life Sciences 5(1): 2160–2167.
Kasture S, Mohan M, Kasture V. 2013. Mucuna pruriens seeds in treatment of Parkinson’s disease: Pharmacological review. Oriental Pharmacy and Experimental Medicine 13: 165–174.
Kosaraju J, Chinni S, Roy PD, Kannan E, Antony AS, Kumar MNS. 2014. Neuroprotective effect of Tinospora cordifolia ethanol extract on 6-hydroxy dopamine induced Parkinsonism. Indian Journal of Pharmacology 46(2): 176–80.
Lieu CA, Venkiteswaran K, Gilmour TP, Rao AN, Petticoffer AC, Gilbert E V. 2012. The Antiparkinsonian and Antidyskinetic mechanisms of Mucuna pruriens in the MPTP-treated nonhuman primate. Evidence-Based Complementary and Alternative Medicine 1:840247.
Mishra AP, Saklani S, Milella L, Tiwari P. 2014. Formulation and evaluation of herbal antioxidant face cream of Nardostachys jatamansi collected from Indian Himalayan region. Asian Pacific Journal of Tropical Biomedicine 4: S679–682.
Mohan M, Yarlagadda S, Chintala S. 2015. Effect of ethanolic extract of Coriandrum sativum L. on tacrine induced orofacial dyskinesia. Indian Journal of Experimental Biology 53(5): 292–296.
Mukherjee D, Ghosal I, Moniruzzaman M, Chakraborty SB. 2017. Methanolic Extract of Mucuna pruriens Seed Acts for Neuroprotection and Antioxidant Defense in A Fish Model. International Journal of Pharmacognosy and Phytochemical Research 9(5): 667-673.
Naidu PS, Kulkarni SK. 2001. Excitatory mechanisms in neuroleptic-induced vacuous chewing movements (VCMs): Possible involvement of calcium and nitric oxide. Behavioural Pharmacology 12(3): 209–216.
Nair SV, Arjuman A, Dorababu P, Gopalakrishna HN, Rao UC, Mohan L. 2007. Effect of NR-ANX-C (a polyherbal formulation) on haloperidol induced catalepsy in albino mice. Indian Journal of Medical Research 126(5): 480–484.
Nakoti SS, Juyal DD, Josh AK. 2017. A review on pharmacognostic and phytochemical study of a plant Nardostachys jatamansi. The Pharma Innovation Journal 6(7): 936–941.
Naqvi E. 2018. Parkinson’s Disease Statistics - Parkinson’s News Today. https://parkinsonsnewstoday.com/parkinsons-disease-statistics/. Accessed 17 March 2020.
Pal A, Kumar M, Saharan V, Bhushan B. 2015. Anti-oxidant and free radical scavenging activity of ashwagandha (Withania somnifera L.) leaves. Journal of Global Biosciences 4(1): 1127-1137.
Patil R, Gadakh R, Gound H, Kasture S. 2011. Antioxidant and anticholiiergic activity of Rubia cordifolia. Pharmacologyonline 2: 272-278.
Patil RA, Hiray YA, Kasture SB. 2012. Reversal of reserpine-induced orofacial dyskinesia and catalepsy by Nardostachys jatamansi. Indian Journal of Pharmacology 44(3): 340–344.
Rane M, Suryawanshi S, Patil R, Aware C, Jadhav R, Gaikwad S. 2019. Exploring the proximate composition, antioxidant, anti-Parkinson’s and anti-inflammatory potential of two neglected and underutilized Mucuna species from India. South African Journal of Botany 124: 304–310.
Ray Dorsey E, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC. 2018. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 17(11): 939–953.
Sahu R, Dhongade HJ, Pandey A, Sahu P, Sahu V, Patel D. 2016. Medicinal properties of nardostachys jatamansi (A Review). Oriental Journal of Chemistry 32: 859–66.
Sharma SK, Singh AP. 2012. In Vitro Antioxidant and Free Radical Scavenging Activity of Nardostachys jatamansi DC. Journal of Acupuncture & Meridian Studies 5(3): 112-118.
Shetty AS, Bhatia KP, Lang AE. 2019. Dystonia and Parkinson’s disease: What is the relationship? Neurobiology of Disease. Academic Press Inc.; vol-132. p. 104462.
Somani RS, Kasture VS, Kasture SB. 1999. Haloperidol inhibits (-) bicuculline-induced seizures and bicuculline potentiates haloperidol-induced catalepsy in mice. Indian Journal of Pharmacology 31(6): 434–436.
Tai YC, Lin CH. 2020. An overview of pain in Parkinson’s disease. Clinical Parkinsonism & Related Disorders 2:1–8.
Tsai G, Goff DC, Chang RW, Flood J, Baer L, Coyle JT. 1998. Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. American Journal of Psychiatry 155(9): 1207-1213.